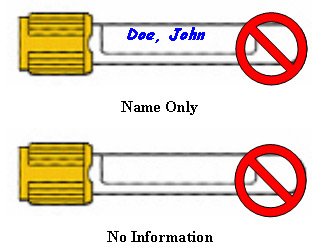
Specimen Identification
CentraCare Laboratory Services (CCLS), in compliance with regulatory requirements, mandates proper specimen identification throughout all phases of laboratory processes. Inappropriate specimen identification can lead to adverse outcomes such as unnecessary or delayed treatments and procedures, delayed diagnosis, and increased cost to the patient and health system.
1. Properly labeled specimens must be labeled in the presence of the patient.
- Specimen containers should not be pre-labeled, with few exceptions (i.e. Take-home collection containers).
- CCLS has adopted and recommends the practice of visual confirmation of specimen identification by the patient post-collection.
2. Properly labeled specimens must include at least two appropriate forms of identification. Commonly accepted forms include:
- Full/Legal Name: (First and Last Name). Nicknames or abbreviated versions do not meet regulatory guidelines.
- Identification Numbers: Medical Record, Chart, etc. Locations/Room Numbers are not considered unique identifiers.
- Date of Birth
- Social Security Numbers
In an effort to standardize processes and limit potential specimen identification errors, the following labeling guidelines have been established. Although the following examples are specific to blood specimens, these practices should be followed with any patient specimens.
Appropriate Labeling Practices
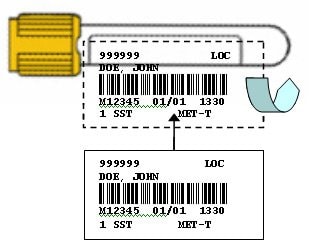
Appropriate Labeling Practices Using Sunquest Labels (CentraCare Laboratories)
- Hold sample tube horizontally with cap in left hand.
- Affix patient label directly over manufacturer label to be read from left to right, starting below tube cap. This allows for:
- Visual inspection of sample by Laboratory for volume and quality
- Consistent, easy sample identification reading by Laboratory
- Reduced potential of re-labeling error in the Laboratory
- Laboratory barcoded labels to be placed over previous labeling without covering original name or interfering with instrumentation
Example:
Appropriate Handwritten Labeling Practices
- Hold sample tube horizontally with cap in left hand.
- Write patient name as close to the top edge of the manufacturer’s label as possible, with identification number or date of birth below. This allows for adherence to appropriate re-labeling practices.
Example:

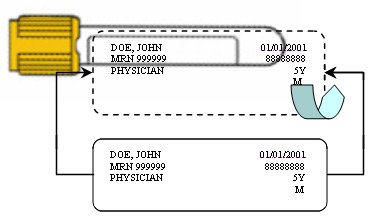
Appropriate Labeling Practices Using Chart/Facility Labels
- Hold sample tube horizontally with cap in left hand.
- Affix patient label directly over manufacturer label to be read from left to right, starting below tube cap. This allows for:
- Visual inspection of sample by Laboratory for volume and quality
- Consistent, easy sample identification reading by Laboratory
- Reduced potential of re-labeling error in the Laboratory
- Laboratory barcoded labels to be placed over previous labeling without covering original name or interfering with instrumentation
Example:
Inappropriate Labeling Resulting in Laboratory and Patient Safety Complications
"Flagging"
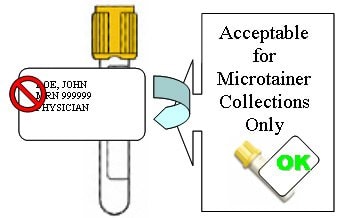
Covering Caps
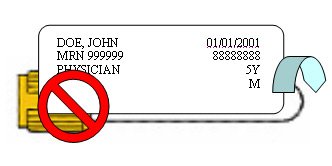
Upside Down
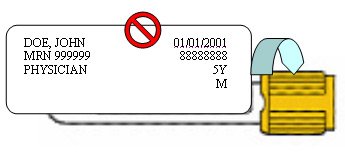
"Coiling"
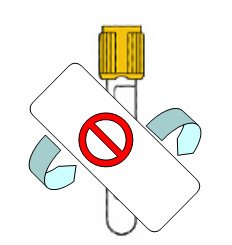
Covering Entire Sample View
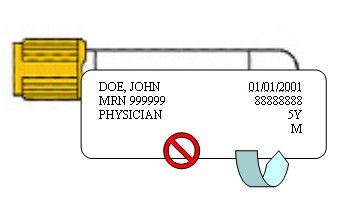
Incompletely Labeled or Unlabeled